1 Introduction Part 1
1.1 Abstract
This chapter explores the devastating illness of tuberculosis (TB) and the growing interest in host-directed therapy (HDT) as a potential avenue for novel anti-TB therapies. We aim to convey how one niche class of human lipids, sphingolipids, may affect the course of a Mycobacterium tuberculosis (Mtb) infection. We introduce TB, its historical context, and the evolution of mycobacteria alongside humans. We also introduce the crucial and enigmatic roles of sphingolipids throughout human biology and how they may present novel ways of treating TB infections. These historical contexts are essential for a deeper discussion on the interplay between Mtb infection and the lipids in the host cell. Further, it is necessary for individuals attempting to discover potential tuberculosis treatments to consider the disease’s historical background. A low-cost, effective therapy is a crucial first step in rectifying the long-standing systemic injustices of tuberculosis treatment.
1.2 Introduction
Tuberculosis (TB) is a devastating illness. The annual WHO Global TB Report estimates that 2 billion people currently host a latent TB infection and that there were 9 million new cases of active TB disease in 20221. In 2022 alone, 1.3 million people died from TB disease – it ranks 14th among overall causes of death worldwide. It is the 2nd leading cause of infectious death worldwide (trailing just behind COVID for the third year running)1. Historical estimates suggest that TB has killed more humans than any other infectious disease2.
In the search for novel anti-TB therapies, there is growing interest in host-directed therapy (HDT), which seeks to synergize with frontline antibiotics by altering the host conditions – making us a less hospitable host – and re-sensitizing the bacteria to antibiotic therapy. Before modern medicine can accomplish this, basic research is needed to understand how Mtb interacts with us as a host organism. As discussed below (and in nearly every chapter of this work), Mtb’s reliance on host lipids may be an ideal avenue for HDT.
The overarching goal of this dissertation is to understand how one niche class of human lipids affects the course of a Mtb infection. These enigmatic lipids are the sphingolipids, which play essential roles throughout human biology: they are crucial in enabling neuronal activity, maintaining membrane integrity, and regulating cellular decisions between life and death, among many other roles throughout the cell.
We have prepared this introduction to the introduction to bridge a gap between the scientific and non-scientific audiences who may read this dissertation. Historical context often needs to be improved in the academic discourse. It provides an excellent point to jump into a more detailed molecular description of sphingolipids during Mycobacterium tuberculosis infection.
1.3 Mycobacterium tuberculosis: an ancient pathogen
Sometimes called the “White Plague” (in contrast to the Black Death), TB is estimated to have killed one billion humans over the last two centuries1. Pulmonary tuberculosis (TB) is a lung infection caused by a family of bacteria called Mycobacterium. While Mycobacterium tuberculosis (Mtb) causes the vast majority of TB in humans today, other Mycobacterium species such as M. bovis, M. canettii, and M. africanum can cause similar illnesses in immunocompromised humans and non-human animals.
Mycobacteria have evolved alongside humans for tens of thousands of years – paleopathology studies have shown that TB disease in humans has existed since at least the Neolithic age 70,000 years ago3. Mycobacterial molecular signatures (chiefly DNA) have been found in the lungs of ancient Egyptian mummies4. Ancient Greek medical literature written by Hippocrates and Soranus of Ephesus vividly describe clinical cases of TB in language still applicable to modern TB infections5,6.
“There is a latent fever which generally begins towards the end of the day and is relieved with the coming of the new day; this is accompanied by much coughing at the beginning and the end of the night, with the discharge of sanious sputa. The sputa are at first bright red, then muddy, then bluish or greenish and finally white and purulent, and either sweet or salt. The voice is either hoarse or high pitched, breathing difficult, cheeks flushed and the rest of the body, ashen colored. The patient is emaciated. In some cases there is a hissing sound or wheezing in the chest; and, as the disease spreads, there is sweating in the upper parts down to the end of the chest. There is a loss of appetite for food. Sometimes there is a feeling of heaviness in the ravaged lung and the patient spits up fiber of it; sometimes there is a stabbing pain due to the ulceration within the chest. The pulse is weak and thick; the tops of the fingers become thick and there is a hooking of the nails. The feet swell up.”
The disease has been so pervasive throughout history that it influenced art and beauty standards(Figure 1.1)7,8. “Consumptives” in the early stages of TB disease are marked by a pale pallor and flushed cheeks, weight loss, and chronic cough – dovetailing neatly with the Victorian feminine ideal: corseted waist, domestic, and frail7. In her 2017 book, -Consumptive Chic: A History of Beauty, Fashion, and Disease-, Carolyn A. Day writes: “Tuberculosis and its accompanying symptoms were construed as the physical manifestation of an inner passion and drive. It was the outward sign of genius and fervour that literally lit the individual, providing the pallid cheek with a glow”9.
“Consumption, I am aware, is a flattering malady.”
Despite these aesthetic “boons” and long co-evolution, tuberculosis is a devastating disease that was a nearly guaranteed death sentence for much of human history10. Some estimates suggest that over 1 billion humans have died from this bacterial infection. Over the millennia, TB treatment has taken many forms, as will be discussed below.
1.4 Galenic humors to Koch’s postulate: A long history of TB therapy
The Greek doctor Galen of Pergamon theorized that the human body contained four “humors”: phlegm, blood, yellow bile, and black bile11. In addition to his advances in anatomical research, patient care, and sanitation, his humoral theory hypothesized that it was the balance of these four essential liquids that defined health – and in disease, a physician must remove the excess humor via bloodletting or purgative (a concoction that induces vomiting)12. Alongside this model of disease was that of the “miasma,” which postulated rank and odorous “bad air” conferred that illness. It was in this context that the Romans prescribed a combined therapy of rich foods (milk, cheese, wine, and the like) and a change of air (long sea voyages and high-altitude escapes)13. However, through the 19th century, it was broadly accepted that there was no actual cure for the consumption. There was merely the temporary relief of symptoms. In many ways, the length of a consumptive’s life following diagnosis aligned to the size of their bank account and social stature: Was the disease discovered early? Could they afford to travel to exotic locales? Could they maintain this lavish, fatty diet10?
The essential components of Greek and Roman TB therapy were maintained as the standard of care through the early 20th century. However, the advent of the sanatorium (a specialized hospital for TB patients) significantly improved the TB therapy paradigm. The first sanatorium facility was formally opened in 1854 by Hermann Brehmer at his sister-in-law’s hydropathic spa in Görbersdorf in Silesia (then part of Prussia, now Sokołowsko, Poland)14–16. Using Brehmer’s example, many notable sanatoria were established over the subsequent century, including the Sanatorium Turban in Davos, Switzerland, the Adirondack Cottage Sanatarium in Sanarac, New York, and the Oregon University Tuberculosis Hospital on Marquam Hill in Portland, Oregon. The University State Tuberculosis Hospital is now the Campus Services Building on the Oregon Health & Science University campus17.
According to contemporary wisdom, these sanatoria provided three primary benefits:
- Rarefaction of the air – which stimulated deep breathing.
- Purification of the air – which rid the patient of miasmas.
- Infrigidation of the air – which was believed to have an antiseptic effect14.
The typical treatment regimen consisted of exercise (some practitioners believed in leisurely exercise to stimulate blood flow to the lungs, while others believed in militaristic exercise to build character) and long “air rests” in reclined chairs and solariums. We can thank the sanatorium for the popularity of the Adirondack chair: these “cure chairs” were believed to be an essential component of the sanatorium panacea18,19. The patients typically received abundant, rich diets consisting of milk, cheese, butter, fatty meats, and wine10,14,20. However, sanatoria regimens were rarely thoroughly relaxing experiences. Sanatoria often employed patients in manual labor to strengthen their character and bodies and prepare them to reintegrate into society21.
The medical establishment of the late 19th and early 20th centuries embraced the sanatorium. Murray describes that the first American sanatorium was founded in 187522. By 1904, there were 115 across America, and by 1952, there were 839 sanatoria in the US22. The true impact of the sanatorium on a patient’s outcome is somewhat unclear, though certainly better than no treatment at all. For certain forms of TB pathology (namely laryngeal tuberculosis), the success of sanatorium treatment was relatively high, and researchers at the time lauded the effectiveness of the sanatorium regimen23:
To be able to change certain disaster to relative or complete recovery by a little skilful (sic.) interference is such a satisfaction that the treatment of laryngeal tuberculosis in sanatoria deserves every consideration.
Other reports also support that the sanatorium regimen did excellently combat the primary symptoms of TB – the cough and sputum, color and wasting, night sweats and fever – all faded within weeks10. However, we must note that many sanatoria only accepted patients of upper-class society – and only those with fair prognoses at that. The “hopeless cases” were excluded from treatment10. Even given these idealized conditions, one survey found that of 1707 admitted to the King Edward VII Sanatorium in Sussex between 1907 and 1914, 751 had died by 1916 – a 44% mortality rate10,24.
Contemporary media held some suspicion toward the sanatorium. In the 1906 George Bernard Shaw stage play, The Doctor’s Dilemma, a character altercates with a doctor about medicine at the time:
“RIDGEON. I know nothing about smallpox. My line is tuberculosis and typhoid and plague. But of course the principle of all vaccines is the same.
SIR PATRICK. Tuberculosis? M-m-m-m! You’ve found out how to cure consumption, eh?
RIDGEON. I believe so.
SIR PATRICK. Ah yes. It’s very interesting. What is it the old cardinal says in Browning’s play? “I have known four and twenty leaders of revolt.” Well, I’ve known over thirty men that found out how to cure consumption. Why do people go on dying of it, Colly? Devilment, I suppose. There was my father’s old friend George Boddington of Sutton Coldfield. He discovered the open-air cure in eighteen-forty. He was ruined and driven out of his practice for only opening the windows; and now we won’t let a consumptive patient have as much as a roof over his head. Oh, it’s very, VERY interesting to an old man.”
Despite these misgivings, groundbreaking TB research was performed at sanatoria, driving infectious disease medicine toward one historically crucial understanding: that diseases such as tuberculosis spread as a contagion – not the product of breathing miasmas or having too-lively a spirit. In 1720, Benjamin Martin published the first clue that TB may disperse via a specific, mysterious “animalcule” infecting the lung25,26. This hypothesis was largely ignored until 168 years later when Robert Koch experimentally verified this in his seminal 1888 work The Etiology of Tuberculosis27. Shortly after this publication, he would propose the now-famous Koch postulates. These are the gold-standard experimental criteria for establishing that certain microbes cause specific diseases. It was at this point that campaigns for public knowledge about TB and its cause began, greatly aiding in limiting the spread of TB in North America and Europe (Figure 1.4)2.
The sanatorium would only begin to decline once the mass production of antibiotics in the 1930s. The history of anti-tubercular therapies was summarized by Iseman in 200229 with the following timeline:
| 1928 | Discovery of penicillin (not effective on Mtb but induced a paradigm shift in treating bacterial infections). |
| 1944 | Discovery of first anti-tubercular antibiotics: streptomycin and para-aminosalicylic acid. |
| 1952 | Roll-out of first therapeutic cocktail: 18-month “triple therapy,” consisting of streptomycin, para-aminosalicylic acid, and isoniazid. |
| 1970s | 9-month course of isoniazid and rifampicin. |
| 1980s | 6-month course of isoniazid, rifampicin, and pyrazinamide. |
| 2020s | 6-9 month course of isoniazid, rifampicin, pyrazinamide, and ethambutol (RIPE) or 4-month course of rifapentine-moxifloxacin, depending on the drug susceptibility of the Mtb strain and toxicity of drugs in the individual patient30,31. |
Currently, the success rate of standard anti-tubercular treatment is impressively high, with total bacterial clearance in about 88% of drug-susceptible cases1. However, recent years have seen a rise in antibiotic-resistant strains of Mtb, threatening the future of these highly effective therapies. The WHO reports that an estimated 410,000 cases of rifampicin-resistant or multi-drug-resistant TB arose in 20221. Numerous reports have shown that prisons in Post-Soviet states (e.g., Russia, Latvia, Azerbaijan) have rates of multi-drug resistant Mtb approaching 16 times those of other countries, warranting a coordinated, multi-national response to limit the spread of these strains32–34.
Given these multi-drug resistant strains, many fundamental biological questions remain about Mycobacterium tuberculosis before improved therapies may be developed. We will discuss these below, but first, we must address a critical feature of the history of tuberculosis treatment: structural discrimination and racism.
1.5 TB, racism, & inequity: an intertwined history
It is impossible to discuss TB epidemiology without addressing systemic inequity in the medical establishment. Tuberculosis has long been and remains to this day a disease of poverty and discrimination. Even in the most literal sense, colonialism drove the spread of tuberculosis: Brynildsrud et al. used genetic lineage mapping to show that colonialist expansion repeatedly expanded the global spread of pathogenic Mtb strains35. Beyond this literal sense, the history of TB treatment is rife with systemic discrimination.
Sanatoria were indisputably tools of segregation, discrimination, and colonialism. Many reports on systematic discrimination have been published in recent years. In one such report, Smith et al. extensively review discrimination in Canadian sanatoria in their 2021 book Tuberculosis Stigma and Racism, Colonialism, and Migration: A Rapid Qualitative Review36. They write that TB was merely one facet of colonialist violence employed against the native populations of Canada. Even while medical institutions would dismiss symptoms and delay TB diagnosis, medical institutions would strictly enforce the separation of TB-infected indigenous people from their communities in manners strikingly similar to the practice of residential schooling. Bennett of the Canadian Broadcasting Company writes about the shipping of hundreds of Inuit TB patients to the Sanatorium on the Mountain in Hamilton, Ontario37:
“The evacuation split families, left parents and children wondering about their loved one’s fate, sometimes for years. Some died and were buried without their families knowledge; others were sent from hospital to hospital without being tracked.”
As Bennett notes in their 2016 article, the mass transfer of Inuit to the Hamilton Sanatorium occurred primarily between the 1940s and the 1960s, following the roll-out of antibiotic therapy. These new therapies rapidly emptied the Hamilton Sanatorium’s 700 beds of white patronage. This general departure provided a unique opportunity for enforced migration and assimilation. In 2016, the CBC also reported the story of a family’s 50-year search for a lost daughter’s grave38. Marieyvonne Alaka died of tuberculosis meningitis (one example of an extra-pulmonary TB infection). Officials buried Alaka’s remains without record after transferring her between multiple medical facilities. She was just one child forcibly removed from her family among hundreds of indigenous children lost within the Canadian medical system in the mid-1900s.
The inequity seeded by Canadian tuberculosis policy for the Inuit people is deeply rooted and continues into the current era. In 2017, LaFreniere et al. found that the incidence rate of TB disease in Inuit communities was 205.8 cases per 100,00039. In contrast, the national rate was 4.9 cases per 100,00039. A 2021 financial analysis by Uppal et al. examined data reported by the Quebec Ministry for Health and Social Services – they found that the 2019 TB incidence rate was 495 cases per 100,000 in the Nunavik community in Northern Quebec40. Uppal et al. further examined the cost-effectiveness of TB screening. They showed that one-off rounds of community screening for TB disease can reduce incidence rates in a community by 13%, and biennial screening could reduce incidence by up to 59%40. These relatively low screening frequencies were substantially more cost-effective than having no screening programs at all40.
Of course, framing these numbers as financially cost-effective dehumanizes the members of these communities as they seek medical attention. However, such reports are crucial first steps toward equitable models of TB treatment and undoing historical injustice. In this, the Canadian government has pledged to take action. In 2019, Canadian Prime Minister Justin Trudeau formally apologized to the Inuit people for the 20th-century tuberculosis policies and promised to rectify the harm done. The Inuit Tuberculosis Elimination Framework, established in 2018, seeks to abolish TB in the Inuit populace by 203041,42.
“For too long, the government’s relationship with Inuit was one of double standards, and of unfair, unequal treatment … While the government was hard at work creating universal healthcare, it was forcing Inuit into settlements where disease and infection ran rampant. … Today, I’m here to offer an official apology for the federal government’s management of tuberculosis in the Arctic from the 1940s to the 1960s.”
Of course, Canada is not alone in historically discriminatory tuberculosis policies. Connolly and Gibson reviewed in 2011 the role that race had in TB disparity in the US between 1900 and 193543. They cite the 1915 US Census Bureau’s national tuberculosis incidence data, which reported that 23 white children per 100,000 died of TB, while the rate among non-white children was 155 per 100,000 – nearly a seven-fold increase43. They write of a 1918 tax passed in Virginia to fund a whites-only Sanatarium in the Blue Ridge Mountains to add to the already-existing “whites-only” Roanoke Catawba Sanatarium. The “colored” Piedmont Sanatorium in Burkeville, VA, was only one of four facilities in the south that would offer beds to black TB patients and charged a two-dollar-a-night fee43. Connolly and Gibson write that the main sentiment for offering a sanatorium to black TB patients was to limit the potential to infect whites43.
These disparities still exist in the US today, even as the rate of TB incidence has substantially reduced. As reported by Schildknect et al., there were fewer than 2500 cases of TB among US-born citizens in 2022 – 30% of these patients were identified as Black, 26% as Hispanic, and 25% as White44. The incidence rates for Black US-born citizens is 1.9 (cases per 100,000), for Hispanic US-born citizens is 1.4, and for White US-born citizens is 0.345. The TB incidence among American Indian or Alaskan Native people is 4.4 cases per 100,000 – over 14-times higher than that of White US-born citizens45. Among Native Hawaiian and other Pacific Islander US-born citizens, the TB incidence rate is 6.6 per 100,00045. As in Canada, much work remains to rectify the inequitable health disparities among minority populations in the US.
Countless examples of systematic racism and colonialist violence in TB treatment exist, and we cannot adequately summarize the scope of this injustice here. However, we must note that modern-day epidemiology continues to follow global trends in poverty and post-colonialism. The 2023 Global Tuberculosis Report by the WHO reports that1. They find that the top eight countries with the highest numbers of total TB cases are all previously colonized nations, amassing over two-thirds of the total cases globally: India (27% of global cases), Indonesia (10%), China (7.1%), the Philippines (7.0%), Pakistan (5.7%), Nigeria (4.5%), Bangladesh (3.6%) and the Democratic Republic of the Congo (3.0%)1. In the same report, the WHO cites that, globally, 50% of all TB patients will incur catastrophic costs for their treatment1 – demonstrating that there is a massive need for reduced cost of treatment for individuals. As will be discussed further below, the most significant risk factors for TB incidence all increase with poverty.
1.6 Mycobacterium tuberculosis today: questions, challenges, and goals
With the increasing prevalence of antibiotic-resistant Mtb strains, many essential open questions about TB disease exist. Chief among these includes: Why do some people with latent TB infections go on to get sick and others do not? How does TB so effectively elude treatment? Are there mechanisms we can use to prevent the onset of infection? We will address some of these questions below. Still, these questions have stood for years, decades, and centuries – the existence of these questions drove much of the research in this dissertation project.
A profound distinction lies between a latent TB infection and an active TB infection. The WHO reports that less than 5% of incipient Mtb infections will go on to produce active disease within two years1. The WHO identified five major risk factors for developing active TB disease (Table 1.2).
In addition, ongoing conflicts, poverty, food and energy insecurity, and the long-term effects of the COVID-19 pandemic indirectly contribute to many more cases.
In observing the list of primary TB risk factors, one may note that many of these factors are known to impose harmful effects on the human immune system. We must emphasize that “hosting a latent TB infection” is no passive state: every Mtb-infected person is waging a war kept at a stalemate for months or years. The human immune system has adapted to fight off most bacterial pathogens we experience daily. It is usually sufficient to destroy and repel would-be invaders. However, Mtb has selectively utilized humans as hosts for millennia, thereby developing a bristling arsenal of molecular tools that block or subvert our typical immune control measures. The above risk factors appear to tip the balance towards -Mtb-. However, it is essential to note that in parallel to -Mtb- acquiring these pathogenic tools, the millennia have allowed humans to develop immune mechanisms to restrict tuberculosis disease progression.
The tug-of-war between bacteria and the immune system allows a latent tuberculosis infection to go unnoticed for months or years in an immunocompetent host: structures called granulomas sequester the bacteria and prevent their dissemination throughout the lung. These structures are composed of immune cells recruited to the site of infection and prevent the bacteria from disseminating throughout the lung. In this encapsulated space, immune cells release anti-microbial factors and inflammatory molecules restricting the bacteria’s growth. Researchers have shown that in some – perhaps many – immunocompetent patients will spontaneously clear an Mtb infection51,52.
Ironically, though, many of the cells recruited to the granuloma are also ideal hosts for Mtb. These cells can become infected and sustain the bacterial population within the granuloma53. Confusingly, individual granulomas within a single host can lie along a spectrum of Mtb burden. Gideon et al. investigated the range of Mtb burdens within individual granulomas in individual macaques. They found that, within one animal, some granulomas may have no living bacteria and will soon heal; in contrast, other granulomas can host thousands of bacteria and show signs of immune failure52. Thus, each granuloma is a disputed battleground in a precarious balance. Any risk factor listed in Table 1.2 above can tip the balance toward active TB disease. Additionally, immunocompromising events such as HIV, chemotherapy, or organ transplant can significantly reduce an individual’s ability to fight a latent TB infection and have high co-mortality with TB.
Because the immune system is essential for restricting active TB disease, many studies have sought to prepare an effective vaccine against Mtb to improve the host response. Robert Koch, the discoverer of the Mycobacterium tuberculosis bacillus, produced the first anti-TB vaccine. He found that a glycerol extraction of cultured Mycobacterium tuberculosis produced a substance, tuberculin, that had a meager ability to immunize against infection. However, tuberculin’s ineffectiveness (a rate of protection less than 5%) and irreproducibility (he kept the formulation of this extract secret for a suspiciously long time) would lead to the end of Koch’s career54,55. The current Mtb vaccine, Bacillus Calmette-Guerin (BCG), is a live attenuated form of Mycobacterium bovis, and has been in use since 1921 and has been widely administered to approximately 5 billion people29,56. The BCG vaccine is estimated to have saved tens of millions of lives55. However, its effectiveness leaves much room for improvement: it is somewhat effective in lessening TB disease in children (~50%), though it has nearly no effect in adults29. There are many ongoing efforts to improve this vaccine to prevent disease onset. However, these efforts face many nuanced challenges, discussed at length by Zhang et al. and others57.
A growing interest in the TB field is in applying so-called host-directed therapies (HDT). The goal of HDT is to indirectly affect Mtb’s survival within an infected patient by altering the patient’s biochemistry to make them a less hospitable host. Many authors have described in detail various possible avenues of HDT in Mtb58–60. In one recent example, Dutta et al. show that in Mtb-infected mice, cholesterol-reducing statins synergized with antibiotic therapy and cleared the Mtb at a significantly enhanced rate. Clinical trials are ongoing to assess the effect of statins on human Mtb infections.
1.7 The lipid connection
Mycobacterium tuberculosis is an intracellular pathogen. Within the lung space, it infects host cells in a Trojan Horse-style infection, allowing itself to be ingested by immune cells called alveolar macrophages (macrophage translates to “big eater” in Greek). These cells are typically the first line of defense against a would-be pathogen. However, Mtb reprograms the macrophage and turns it into a suitable host for replication. Accordingly, many have described Mtb as a “master manipulator” of host biology61,62.
As a pathogen living within host cells, Mtb must acquire all its nutrients and implement all its machinations through lipid membranes. Mtb appears to be highly attuned to host lipid biology, leading to new potential routes of antitubercular therapy.
In this chapter and the next, we will extensively discuss the intricate ties between host lipid biology and an infecting Mtb bacillus. First, though, we have to step back to look at what lipids are.
1.7.0.1 So what is a lipid?
Lipids are one of the main four macromolecules in biology. We will describe these macromolecules in the order of the central dogma – the direction that information flows in life on Earth. The first biological macromolecule is nucleic acid, which comes in the form of DNA and RNA in the human body. DNA encodes all the heritable genetic information within every cell within the human body (as well as all other plants, animals, fungi, bacteria, and many viruses on Earth) and serves as the permanent record of all information in the cell. RNA is transcribed from DNA as needed to serve as a short-term blueprint for immediate use in the cell – a quick printout of the instructions for the cell to follow, quickly destroyed but easily remade. The third macromolecule is protein, which is a sequence of amino acids translated from the RNA blueprint – these long chains of amino acids are carefully folded into precise three-dimensional structures with catalytic clefts, binding sites, and hydrophobic domains that combine to allow each protein to perform a specific chemical reaction or interaction in the cell. Proteins mediate the production of the third and fourth macromolecules: carbohydrates and lipids. Carbohydrates encompass the sugars that serve as rapid energy sources and the cellulose made by plants for structural rigidity and chitin in the shells of crabs and other arthropods. Finally, lipids are the (relatively) small, greasy molecules commonly known as fats – they serve as long-term energy storage, are the root structure of many hormones, and, most importantly, self-assemble into membranes.
At its most fundamental, a membrane defines “inside” and “outside” – these are the crucial barriers that allow a cell to perform chemistry. We can simplify every cell on Earth down to a tiny compartment enclosing a concert of chemical reactions. The walls delineated by these compartments are lipid membranes. Without these membranes, it would be impossible to amass chemical inputs and outputs, store energy, and build ion gradients – the fundamental processes underlying all life on Earth.
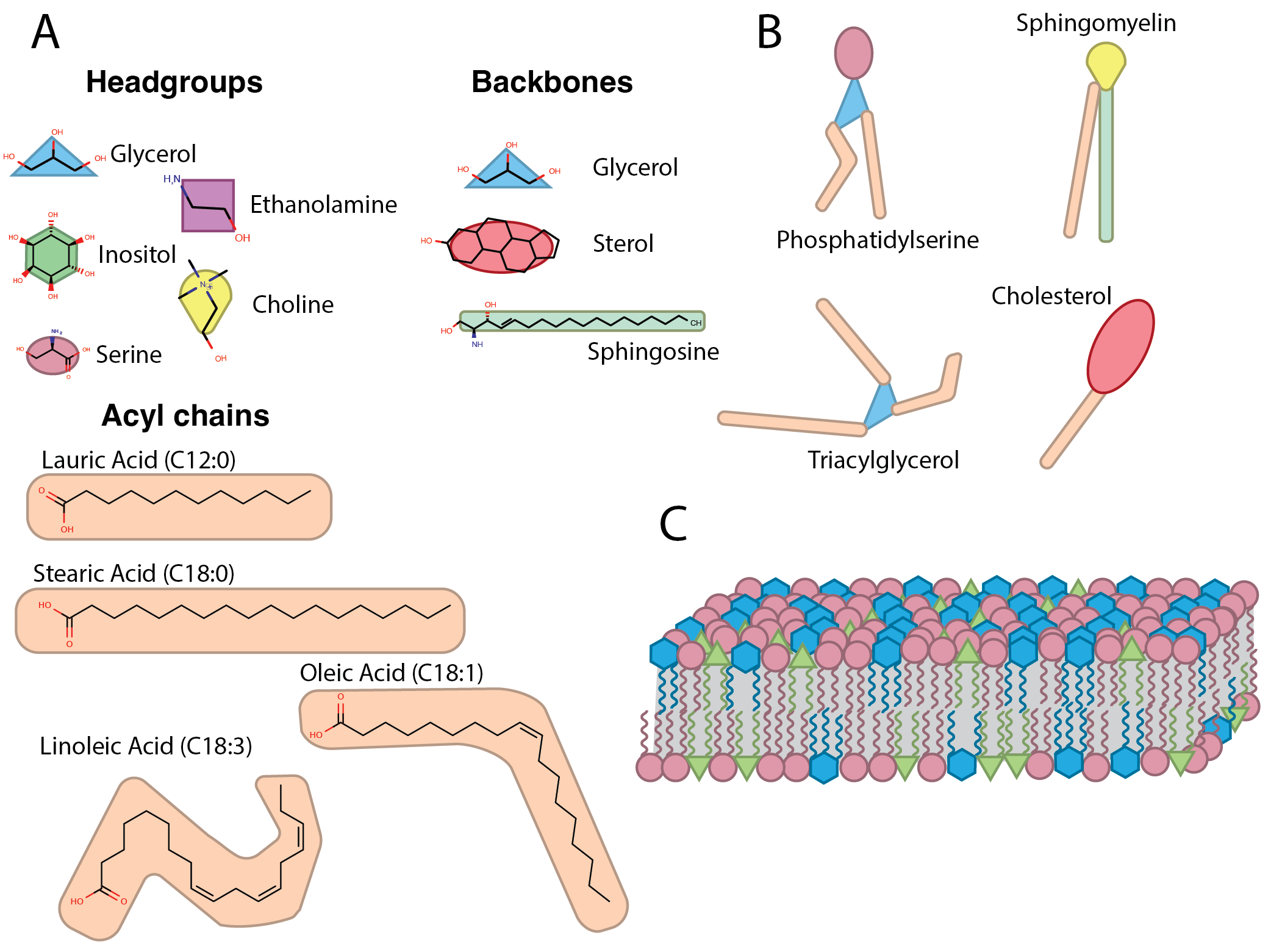
All biological membranes are primarily composed of lipids, which all have a broad similarity: a hydrophilic structure that preferentially interacts with water (the “head” or “headgroup”), a hydrophobic structure (the “tail”) that preferentially avoids interaction with water by aggregating with other hydrophobic molecules, and a backbone structure, which links the headgroup with the tail (Figure 1.6A). In this manner, lipid membranes self-assemble in an aqueous solution into bilayers with headgroups facing out into the water and tails shielding each other from the water (Figure 1.6C)63.
A human cell has thousands of molecularly distinct lipids (“species”). There are five major lipid headgroups: glycerol, sterol, inositol, ethanolamine, and serine (and many modifications of these core structures, such as phosphorylation and glycosylation); there are also many possible lengths of the hydrophobic tail and many sites of possible desaturation (Figure 1.6B). Lipid-metabolizing enzymes mediate the combinatorial addition of these components in the cell to produce a vast mosaic for membrane composition(Figure 1.6C).
For much of the history of biological research, lipids were considered the inert backdrop upon which the cell performed its chemical act – just the walls and roof that proteins (the real stars of the show) used as a stage. However, researchers are increasingly recognizing lipids for the nuanced and dynamic cadre they are. These molecules are synthesized in precise proportions, distributed in careful patterns throughout membranes, and induce potent effects in the cell – how could anyone have thought these were random and inert molecules?
Careful studies have shown that each compartment in the cell is defined by a membrane with distinct properties64. For example, a highly fluid membrane encloses the endoplasmic reticulum, which must flow dynamically to traffic freshly synthesized proteins throughout the cell; accordingly, the ER membrane is primarily composed of phospholipids and contains virtually no sterols, cardiolipins, or complex sphingolipids which confer rigidity.63. In contrast, the outer membrane of the cell (the plasma membrane) needs more structural rigidity to withstand shear forces and protect the cell; this membrane is composed of less-fluid lipids that aggregate in structurally rigid microdomains such as cholesterol and sphingomyelin63. Mitochondria need even more structural stability and insulation because their role as the cellular powerplant requires the production of steep electrochemical gradients and reactive molecules such as peroxides; these organelles are encased in a rigid double membrane composed of cardiolipins65,66. The containment of the mitochondrial contents is so crucial to cell survival that the mitochondria serve as a self-destruct button for the cell: controlled cell death (i.e., apoptosis) relies on permeabilizing the mitochondrial membrane to trigger a reactor meltdown66.
Even within a single membrane, there can be tight, localized regulation of lipid composition: the plasma membrane lipid bilayer is asymmetrically composed of negatively charged lipids on the inner leaflet (mainly phosphatidylserine, PS) and neutral lipids on the outer leaflet of the bilayer. This asymmetry encourages the transfer of select ions into the cell and can serve as an instant reporter of cell damage for surveilling immune cells. When a cell dies, it actively flops PS from the inner leaflet of the plasma membrane to the outer leaflet, transferring negative charges to the outside of the cell; nearby macrophages (cells that we will discuss in much more detail in the following chapter) have receptors that recognize this outside-facing PS and immediately clear the dead cell away so a neighbor can take its place.
1.7.0.2 What do lipids have to do with TB?
In many ways, Mycobacterium tuberculosis is a bacterium distinguished by its unique lipid composition and sensitivity to environmental lipids.
Garcia-Vilanova et al. discuss how Mtb’s lipid composition appears to change between the intracellular stages of the Mtb lifecycle versus the airborne, transmissible stage62: it enriches itself of diacyl trehalose, triacyl trehalose, phthiocerol dimycocerosate and other waxy lipids appears to confer strong hydrophobicity. These waxy and greasy cell wall lipids confer Mtb’s astounding ability to stay in aerosols (the essential transmission mechanism for TB)67. Within the lung space, Mtb alters its lipid coating toward a cohort of lipids known to induce pathology: it up-regulates the production of phosphatidyl-myo-inositol mannosides (PIMs) and mannose-capped lipoarabinomannan (ManLAM)62. These waxy coats are an essential source of difficulty in the search for rapid anti-TB antibiotics. This greasy coating physically excludes many molecules and protects Mtb from many antimicrobial toxins62.
In another example, Mtb is known to induce massive changes to the host lipid landscape during infection. So-called “foamy” macrophages in the lung are a clinical sign of an active TB infection. These infected macrophages are replete with structures called lipid droplets (visually appearing as a foam inside the cell) and typically serve as energy storage deposits for lipids such as triacylglycerol. Much work has shown that Mtb infection drives the production of these lipid droplets68 and the selective delivery of these structures to the Mtb-containing compartment69. These dramatic changes to the host lipid composition are part of why Mtb is called a master manipulator of host biology61.
Host lipids also play a critical role in driving the bacteria along the drug-resistant versus drug-susceptible axis. As referenced above, Dutta et al. showed that Mtb-infected mice treated with cholesterol-reducing statins are highly responsive to antibiotic treatment and resolve their Mtb infections much more quickly than control mice70. This possible avenue for host-directed therapy has drawn significant attention, and ongoing clinical trials seek to discover its effect in humans. What other lipids may influence a TB infection?
1.8 Sphingolipids: enigmatic like the sphinx
Sphingolipids are a family of lipids with a central chemical structure, the sphingoid base. These lipids were named by Johann Ludwig Wilhelm Thudichum in 1884 because they are “as enigmatic as the sphinx.” Of course, this has been the source of more than a lifetime’s worth of embarrassing paper titles, including: Taming the Sphinx: Mechanisms of cellular homeostasis71; HDL Dysfunction: Is the Answer in the Sphinx’s Riddle?72; Sphingosine-1-Phosphate and Macrophage Biology—How the Sphinx Tames the Big Eater73; The Squeaky Yeast Gets Greased: The Roles of Host Lipids in the Clearance of Pathogenic Fungi (See Chapter 8)74.
Until quite recently, biologists knew very little about what sphingolipids do. We now know that sphingolipids play critical roles in immune signaling, nervous system function, brain development, and apoptosis. The dysregulation of sphingolipid production and metabolism is associated with various cancers79–81, immune disorders82,83, and bone disease84, among many, many other diseases81.
Sphingolipids play an intricate role in a cell deciding between life and death. The so-called “sphingolipid rheostat” is a well-described program in which the proportion of ceramide versus sphingosine tips the cell toward death versus life (respectively). At first glance, this seems counterintuitive: why should a cell actively initiate its own death? However, programmed cell death (apoptosis) is critical in multicellular organisms: when a cell senses that it is irreparably damaged or infected by a pathogen, it must be destroyed and replaced for the good of the whole organism85. For this reason, deficiencies in sphingolipid regulation are tightly associated with neurological disease, autoimmune disorders, and cancer80,86,87. Recent work even suggests that sphingolipids may play crucial roles in aging and aging-related diseases88.
We have also previously written on the ways that sphingolipids have been shown as critical determinants of microbial pathogenesis (see Chapter 7 and Chapter 8). These lipids play crucial roles in nearly every context in which they’ve been investigated. Despite this, much research remains to uncover how sphingolipids influence a Mycobacterium tuberculosis infection. The ties that sphingolipids have to cell death, membrane repair, membrane structure, and immune signaling are all highly intriguing connections between sphingolipids and Mtb. Throughout this document, we will discuss these connections in great detail.
Some researchers have ventured beyond entirely scientific avenues to philosophize that the tight regulation apparent in the sphingolipid pathway serves as evidence of “fine-tuning” – the notion of inexplicable sensitivity to perturbation which belies a grand universal architecture and hints toward directed evolution89. These authors point to this pathway, which decides the life and death of the cell by the interconversion of a few mol% of one molecule into another, and dismay that it could not arise spontaneously. That is not to say there is no awe to feel when thinking about the vast complexity of biological systems. Imagine thousands of unique sphingolipid species dancing in swirling membranous majesty within every cell of your body, triggering cell death or disease with any stumble. But now, consider that this pathway predates the separation of eukaryotes from prokaryotes90. The tight regulation of sphingolipids is the product of 2.5 billion years of evolution. 2.5 billion years ago, the Moon had active volcanos and was visibly closer to the Earth91,92. A day was 17 hours long (because the Moon has been gently slowing the rotation of the Earth)92. Saturn did not yet have rings93. The rise of photosynthesis would soon cause the Great Oxygenation Event and kill the majority of all life on Earth94. This span of these aeons should inspire at least as much awe as the complexity of a membrane. The fact that sphingolipids are so intricately tied to cell life and death merely highlights the strength of the evolutionary pressures that produced this self-regulating network. In short, fine-tunedness is as controversial in biology as in cosmology.
1.9 Conclusion
Tuberculosis is a complex, sometimes self-contradictory disease. Sphingolipids are a complex, sometimes self-contradictory family of lipids. Accordingly, this introduction has rambled and sprawled over many topics and only scraped the surface of many detailed processes.
We hope this chapter has provided some historical context and introduced vital concepts we will expand upon as the following chapters increase in technical detail.
1.10 Contributions
GG compiled the concepts discussed in this article and wrote the entirety of the text. FGT oversaw the preparation of this article.